Gerneral Information about Pyrrolizidine Alkaloids
Introduction
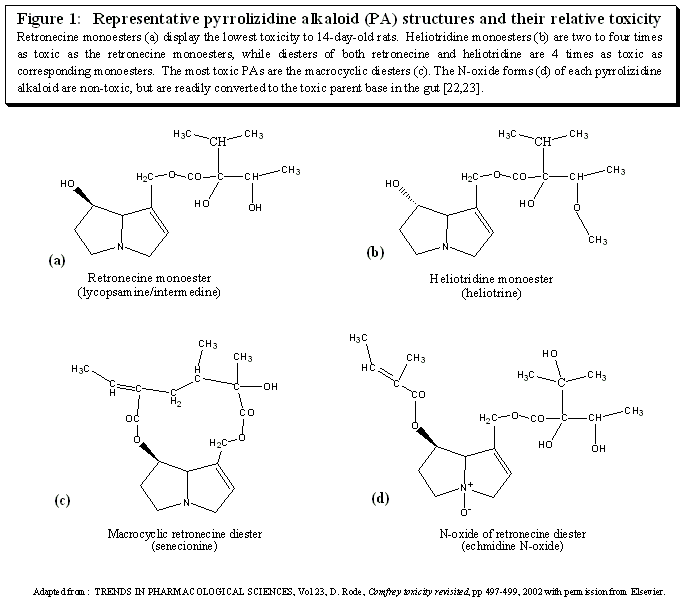
Compounds with the characteristic structure depicted in figure 1 are classified as pyrrolizidine alkaloids. These compounds are widely present in plants with as many as 6000 species containing them[18]. However only about half of the identified PAs are toxic. Within the subset of dangerous PAs the type and extent of the toxicity depends on the specific PA, the stability of the toxic metabolite (pyrrole) that is produced from it, the rate at which the reactive metabolite is produced, the species and sex of the exposed subject, the dose, the route of administration and the health/nutritional status of the test subject. In considering crude plant material, toxicity also depends on the plant part used (root, leaf, etc.), the growth stage of the plant, and the individual plant used. In addition, PA activity in general may be reduced after long-term storage[26].
 |
Toxicity
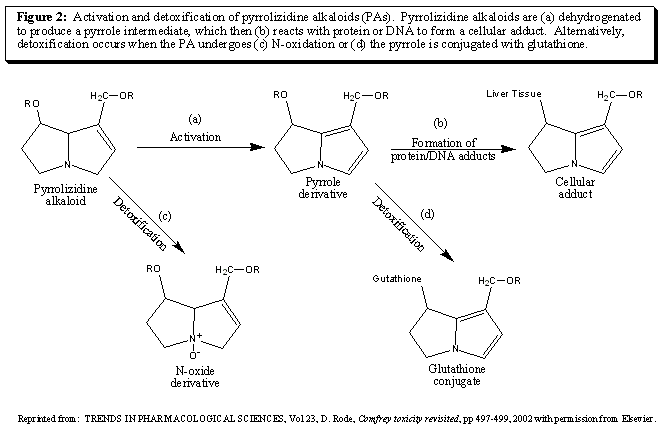
Technically, PAs are not toxic in themselves. They are transformed in the liver by mixed function oxidases to pyrroles which exert their toxic effect by reacting with and binding to cellular macromolecules, including proteins and DNA [18, 20] (figure 2). These cellular adducts produce acute toxicity and purposively are retained by the liver resulting in chronic tissue changes [20,21]. However, it is possible that the PA or its active metabolite could be detoxified by the liver into a more soluble form and safely excreted in the urine (figure 2). In addition, less reactive species at higher concentrations can be released from the liver and damage other tissues (i.e. lungs)[20, 24]. In addition, there is evidence that metabolites other than pyrroles may also play a role in the toxic effects of PAs [25].
 |
The structure of each PA defines the stability of the corresponding toxic metabolite (the pyrrole) and its potential for inducing damage. A general relationship between structure and toxicity has been elucidated (figure 1).
- Macrocyclic diesters are the most hepatotoxic pyrrolizidine alkaloids [22].
- Heliotridine esters are 2 to 4 times as toxic as retronecine esters [23].
- Diesters of heliotridine and retronecine are 4 times as toxic as their respective monoesters[23].
It is important to note that these structure/toxicity relationships place comfrey PAs (retronecine mono and diesters) in a class with lower toxicity than the PAs implicated in significant human poisonings. The heliotridine diesters and macrocyclic diesters of retronecine found in plants such as Senecio, Heliotropium, and Crotolaria have been responsible for tragic poisonings when inappropriately used in medicine or inadvertently contaminating food [22].
Veno-Occlusive Disease (VOD)
The PA toxicity observed in humans manifests as veno-occlusive disease (VOD), characterized by obliterative lesions of the centrilobular and sublobular hepatic veins giving rise to hepatic congestion, hepatomegaly, and ascites [27,28]. Limited lesions are usually followed by complete recovery, while extensive lesions may result in death [29]. VOD sometimes occurs in conjunction with conditions involving immune dysregulation[30, 31] or can be caused by the use of oral contraceptives[32, 33] and other pharmaceuticals[34, 35], especially neoplastic agents [27, 28, 34, 36-39] radiation [28], or the treatment given in conjunction with bone marrow transplants [28, 38].
Species Differ in Response to the Same PAs
The characteristics of PA toxicity in animals can differ tremendously between species and from that of humans[20, 40]. For example the development of megalocytosis, large cells where mitosis appears to be inhibited, is observed in rats, mice, sheep, horses, pigs and trout but not in human cases of PA poisoning[20]. Also, while mice and sheep are resistant to poisoning by Senecio (a PA containing plant), pigs and chickens are highly sensitive (table 1). And sheep and goats can graze pastures made unsuitable to horses and cattle due to the presence of PA containing plants [18].
| Table 1: Comparative ranking of animals based on susceptibility to poisoning by Senecio jacobaea. | ||
| Animal | Chronic lethal dose (% of body weight) | Refs. |
| Pig | 1* | [20] |
| Cow | 4 | [13] |
| Chicken | 5 | [20] |
| Horse | 7 | [13] |
| Rat | 21 | [13] |
| Rabbit | 115 | [13] |
| Guinea Pig | 119 | [13] |
| Mouse | 150 | [20] |
| Goat | 205 | [13] |
| Sheep | 302 | [13] |
| Hamster | 338 | [13] |
| Gerbils | >3650 | [13] |
| * Pig susceptibility is based on Crotalaria retusa poisoning. | ||
| Reprinted from: TRENDS IN PHARMACOLOGICAL SCIENCES, Vol 23, D. Rode, Comfrey toxicity revisited, pp 497-499, 2002 with permission from Elsevier. | ||
The Same Species Respond Differently to Different PAs
The response of a species to a particular PA containing plant may not be predictive of that animal's susceptibility to other PAs. For example, guinea pigs are resistant to monocrotoline and susceptible to Senecio PAs [22]. And rabbits, pigs and chickens are all relatively resistant to comfrey PA toxicity, while rats appear to be more susceptible [87, 101-103]. Additionally, injected PA may lead to increased toxicity when compared to orally administered PAs. This could be due to overwhelming detoxification pathways when the PAs are delivered to the liver in a bolus. For example, rabbits succumb to a single injection of purified Senecio alkaloids while they are relatively resistant to chronic feeding of the plant [41].
Gender and Age Differences
Gender and age differences in susceptibility to PA poisoning have also been reported. Female rats appear to be more resistant than males [26] and nursing dams given doses of PAs at levels that failed to induce liver damage, resulted in fatal liver disease in the majority of their young [18, 44]. In fact, a screening test for PA toxicity takes advantage of the increased susceptibility of the young by using 2 week old unweaned rats [45].
N-Oxides of PAs
PAs are found in plants as either the parent base or the N-oxide derivative (figure 1e). The herbaceous parts of plants typically have more of the PAs as the N-oxide [20, 46]. About 80% of PAs in leaf, root, and stem of Crotalaria retusa was found as the N-oxide [47]. PA N-oxides are more water soluble than their parent alkaloids, are less likely to be metabolized to toxic pyrroles and are quickly removed from the body by the kidney. However, N-oxides appear to be transformed in the gut to the parent base. So while a PA such as retrorsine in the N-oxide form given i.p. (intraperitioneal) will be one fifth as toxic as its parent base, if given orally it will have the same toxicity as the parent base [48]. In addition, N-oxides administered i.p. or i.v.(intravenous) diffuse into the gut, undergo microbial conversion and are absorbed as the parent base[49].
Absorption through the Skin
The extent that PAs are absorbed by the skin was explored in one limited study (two rats per group) using comfrey PAs and measuring urine excretion. Absorption by the skin results in about 20 times less metabolites in the urine than when administered orally. In addition, dermally administered PA N-Oxides of Symphytum were not converted to the parent base, while oral administration resulted in detectable amounts of the parent base in the urine[50]. Topical use of comfrey.
Other Factors Influence Toxicity
The extent of toxicity also depends on the nutritional status of the test subject. Rats fed a low protein diet exhibited higher mortality than those fed a normal diet [51]. Supplementation with cysteine or methionine, which boost the liver stores of glutathione (GSH), is also hepatoprotective[52]. It appears that the reactive pyrrole binds GSH rather than cellular proteins and DNA. These GSH conjugates are much lower in toxicity than the free pyrroles and are excreted in the bile[53]. Taurine, an amino acid found in meat and to a lesser extent dairy, stimulates the enzyme that produces GSH and antagonizes PA induced changes in the conjugation enzyme. Taurine has been shown to protect against PA induced tissue changes and mortality [54].
Since the extent of toxicity is dependent on the production of reactive pyrroles by the cytochrome P450 monooxygenases, selective induction or inhibition of P450s by drugs or food may lead to changes in toxicity. Research has shown that direct inhibition of P450s leads to decreased toxicity and decreased DNA cross-linking[55, 56]. The converse, phenobarbital induction of P450, leads to increased hepatic damage by the PA retrorsine in mice and guinea pigs [57] and heliotrine in rats [58], but affords protection when rats are challenged with a lethal dose of lasiocarpine [58]. Similarily, phenobarbital pretreatment had no effect on the amount of DNA cross-linking induced by the PA monocrotaline in rats [56]. The variability in effect of phenobarbital pretreatment may be due to the simultaneous induction of detoxification pathways. When the normal rate of conversion of PA to pyrrole is low, phenobarbital pretreatment increases toxicity, but when the PA to pyrrole conversion is normally fast, induction of P450 decreases toxicity [20].
N-Indicine, a PA that initially showed promise as a chemotherapeutic agent in cancer because of the absence of hepatic-toxicity, gave rise to a high incidence of VOD when used in the treatment of leukemia in phase II clinical trials even when the dosing regime was identical to that used on patients with solid tumors where only slight and reversible alterations in hepatic function markers were noted [39, 59]. Liver toxicity was also noted in pediatric populations that had undergone extensive prior chemotherapy[39]. These findings suggest that the health status of the individual may effect the observed toxicity of a particular PA with individuals compromised by prior drug treatment being more susceptible.
© 2004 Dorena Rode Acknowledgment